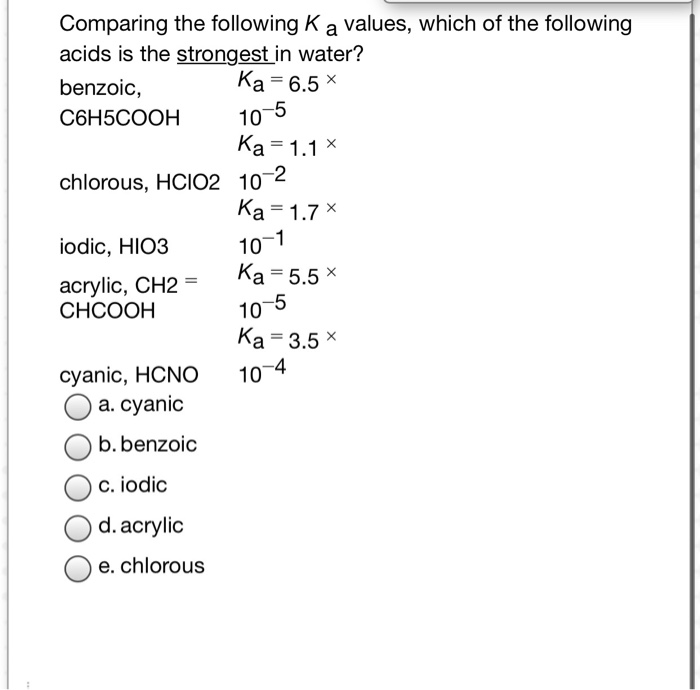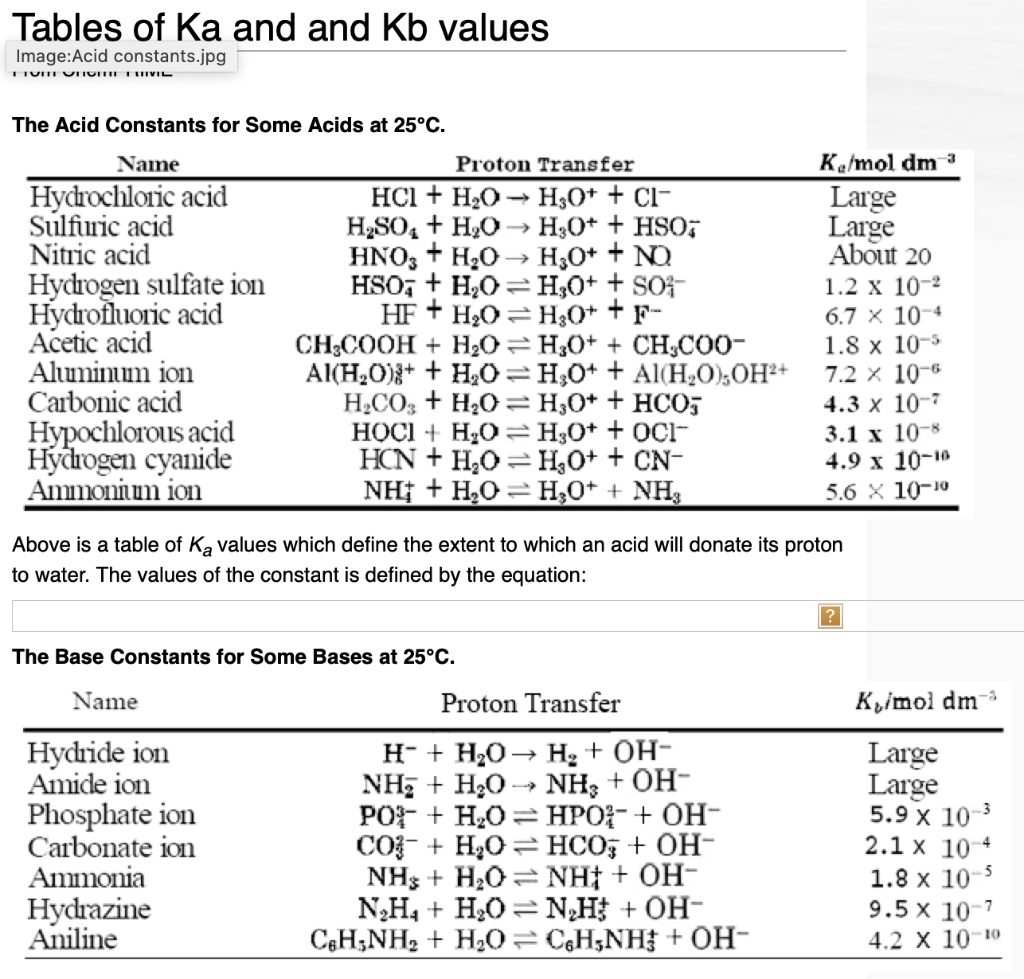
SOLVED: Tables of Ka and Kb values The Acid Constants for Some Acids at 25°C Name Proton Transfer Ka (mol dm^-3) Hydrochloric acid HCI + H2O â†' H3O+ + Cl- Large Sulfuric
Find out the Value ofequilibrium constant for the following reaction at 298 K, 2 NH3(g) + CO2 ⇌ NH2CONH2(aq) + H2O(I) - Sarthaks eConnect | Largest Online Education Community

The value of K_(p) for the water gas reaction, CO +H_(2)O hArr CO_(2) +H_(2)is 1.06 xx 10^(5) at... - YouTube

The value of Kc = 4.24 at 800K for the reaction CO(g) + H2O(g) CO2(g) + H2(g) Calculate equilibrium concentrations of CO2,H2,CO and H2O at 800K , if only CO and H2O
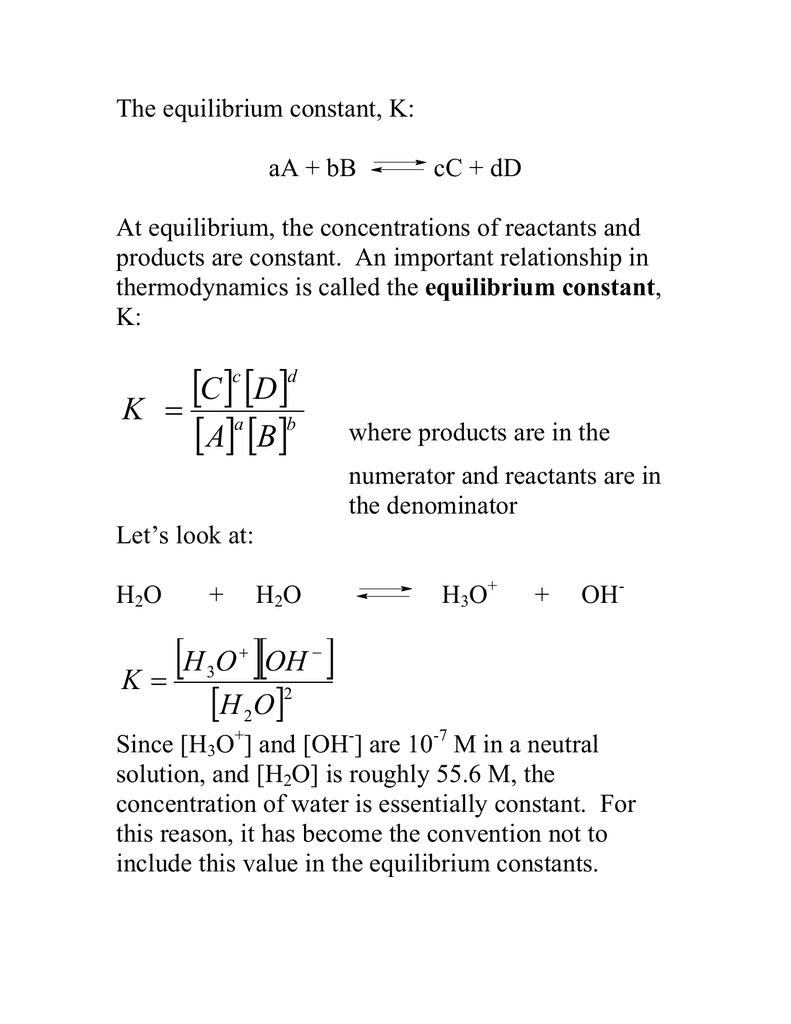
What is value of Ka and kb for water (H20) and how numerically prove that ka ×kb=kw in case of water? - Quora

OneClass: please help with part F Consider the following reaction. H2O(g) + Cl2O(f) 2 HOCI(g) K298 - ...
18. Standard free gibbs energies of formation at 298K are 237.2, 394.4 and 8.2 for H2O, CO2 and pentane respectively. The value of E^° for the pentan oxygen fuel cell is 1) 0.0968V 2)1.968V 3) 2.0968V 4) 1.0968V
the s†an dard enthalpy of formation of gaseous H2O at 298K is 241.82KJ/mol. Estimatr its value of 373K given the following values of the molar heat capacities at cons†an t pressure: H2O(g):35.58J/Kmol,



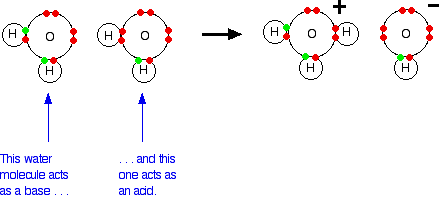
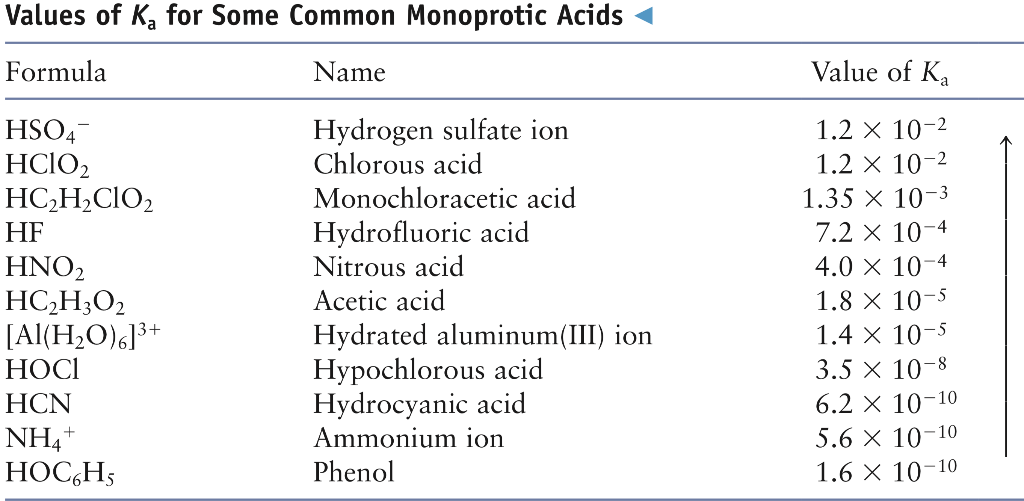
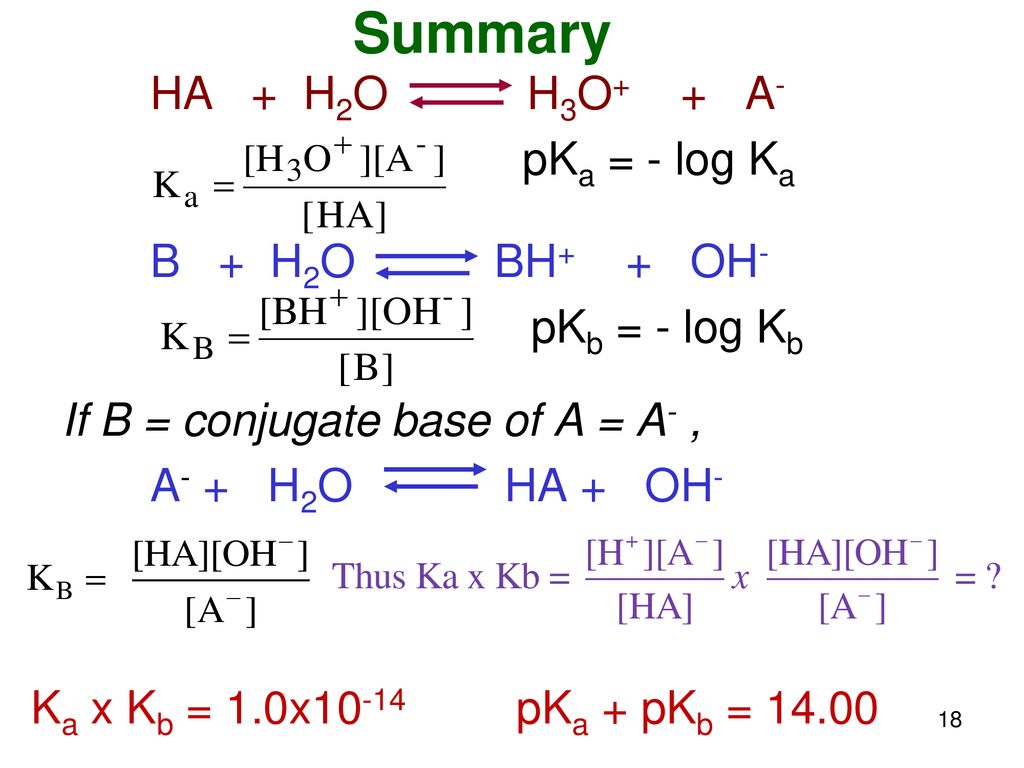

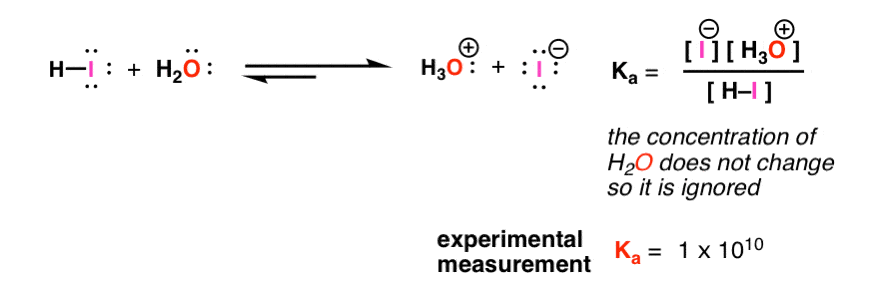



![ANSWERED] A buffer solution contains dissolved C6H5... - Physical Chemistry ANSWERED] A buffer solution contains dissolved C6H5... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/43165458-1658605548.8452456.jpeg)