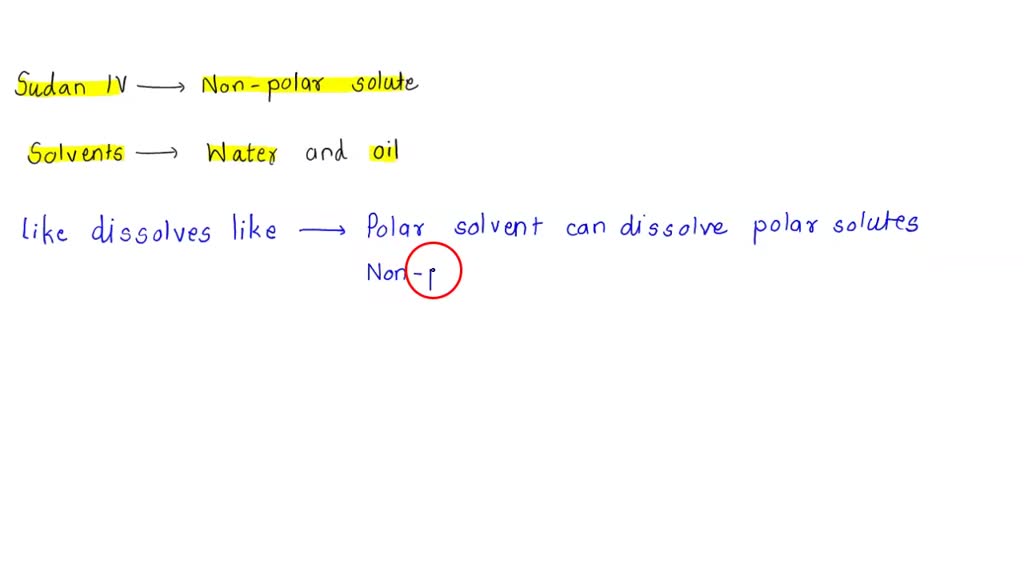
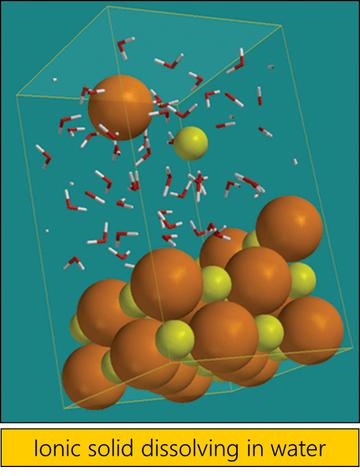
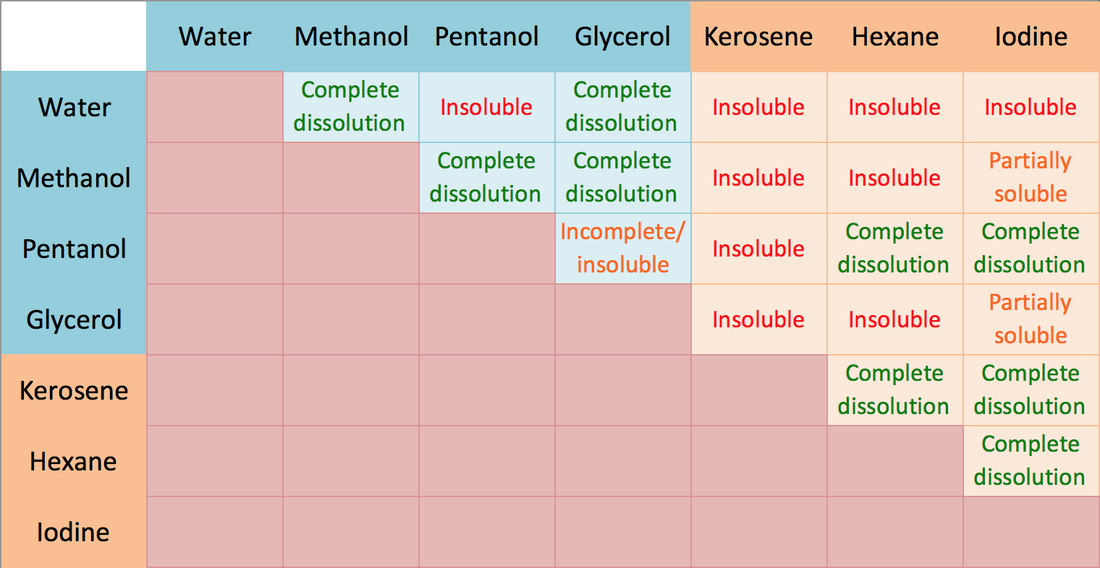
Question Video: Identifying the Correct Relationships between Polar and Nonpolar Solvents and Solutes | Nagwa

Can Liquids Dissolve in Water? | Chapter 5: The Water Molecule and Dissolving | Middle School Chemistry

VIMS Academy JAMMU - Solubility of some polar and non polar solvents by Miss Palvi Gupta Senior faculty of Chemistry VIMS Academy. For more information and knowledgeable content do follow us on

SOLVED: Which of the following will dissolve in polar solvents? non-polar solvents and ionic compounds ionic compounds and polar covalent compounds non-polar solvents and covalent compounds all of the above